Patient Trend and History - LDL-C Level, Medications, and Initiatives
EXPECTED LDL CHOLESTEROL REDUCTION
| PATIENT TYPE | STATIN TOLERANT | STATIN INTOLERANCE | ||
|---|---|---|---|---|
| Achievable Reductions | ≥60% | ≥80% | ≥35% | ≥60% |
| Potential Combinations | Rosuvastatin 20-40 + Ezetimibe 10 | Rosuvastatin 20-40 + Alirocumab/Evolocumab (+Ezetimibe 10) | Ezetimibe 10 + Bile acids | |
| Atorvastatin 40-80 + Ezetimibe 10 | Atorvastatin 40-80 + Alirocumab/Evolocumab (+Ezetimibe 10) | Ezetimibe 10 + Alirocumab/Evolocumab | ||
| Rosuvastatin 40-80 + Ezetimibe 10 | ||||
| Atorvastatin 10-20 + Alirocumab/Evolocumab | ||||
| Rosuvastatin 10-20 + Inclisiran | Rosuvastatin 20-40 + Inclisiran (+Ezetimibe 10) | Bempedoic acid 180+ Ezetimibe 10 | ||
| Atorvastatin 10-20 + Inclisiran | Rosuvastatin 20-40 + Inclisiran (+Ezetimibe 10) | Bempedoic acid 180+ Ezetimibe 10+ Evolocumab, Alirocumab or Inclisiran | ||
| Atorvastatin 20+ Ezetimibe 10+ Bempedoic acid 180 | Ezetimibe 10+ Inclisiran |
References:
- Medicine for High Cholesterol. Food And Drug Administration Philippines website. https://verification.fda.gov.ph/med_high_cholesterollist.php?start=141. Last accessed September 2025.
- Ray, K. K., Ference, B. A., Séverin, T., Blom, D., Nicholls, S. J., Shiba, M. H., … Santos, R. D. (2022). World Heart Federation Cholesterol Roadmap 2022. Global Heart, 17(1), 75. https://doi.org/10.5334/gh.1154
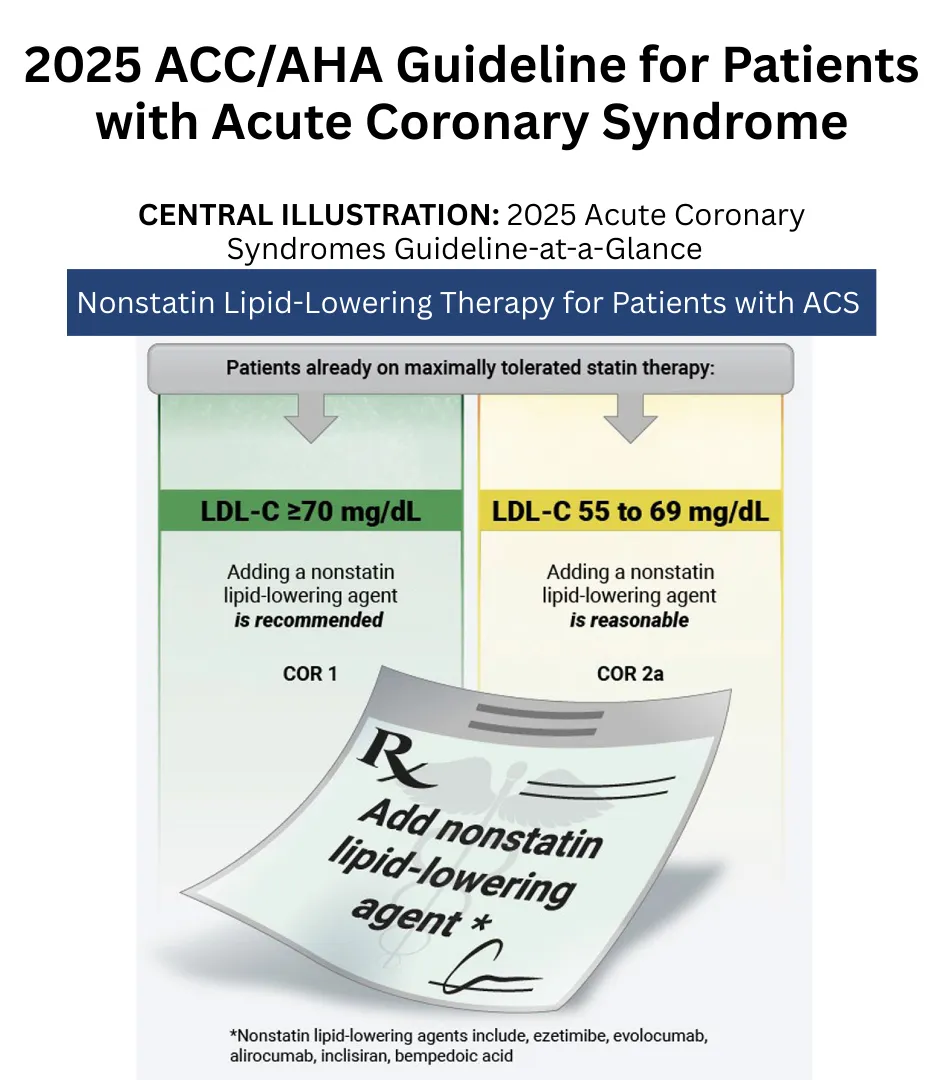
- Kumbhani DJ, Cibotti-Sun M, Moore MM. 2025 Acute Coronary Syndromes Guideline-at-a-Glance. J Am Coll Cardiol. 2025 Jun 10;85(22):2128-2134. doi: 10.1016/j.jacc.2025.01.018. Epub 2025 Feb 27. PMID: 40013745.
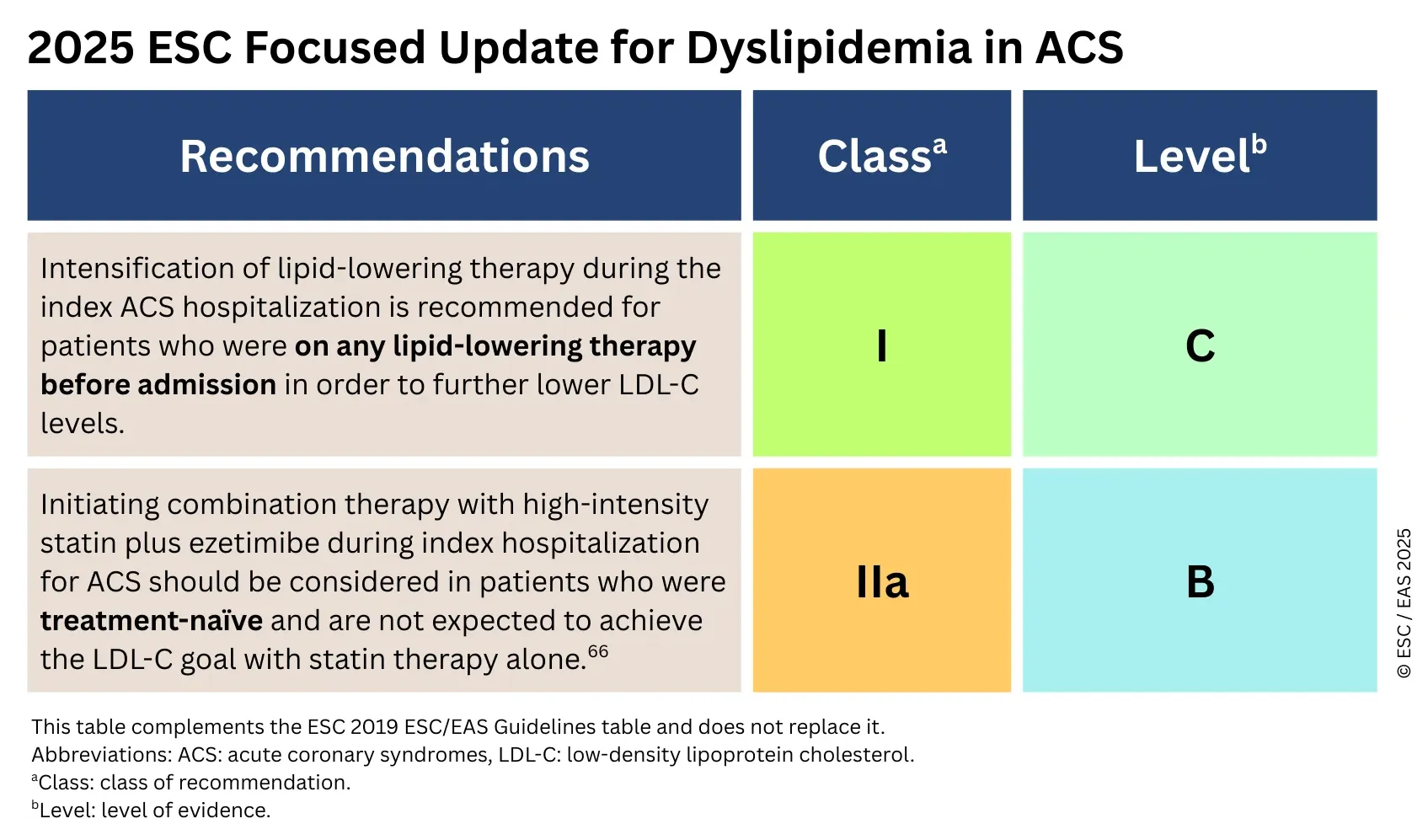
- Mach F, Koskinas KC, Roeters van Lennep JE, Tokgözoğlu L, Badimon L, Baigent C, Benn M, Binder CJ, Catapano AL, De Backer GG, Delgado V, Fabin N, Ference BA, Graham IM, Landmesser U, Laufs U, Mihaylova B, Nordestgaard BG, Richter DJ, Sabatine MS; ESC/EAS Scientific Document Group. 2025 Focused Update of the 2019 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2025 Aug 29:ehaf190. doi: 10.1093/eurheartj/ehaf190. Epub ahead of print. PMID: 40878289.
For Healthcare Professionals only.
The content hosted on this platform is intended for non-promotional scientific purposes only and the content of the site is accurate at the time of publication.
Any data about non-Novartis products are based on publicly available information.
Novartis cannot make individual patient treatment recommendations. A treatment decision has to be made by the treating physician on a case-by-case basis after careful evaluation of the associated benefits and risks.
For the purposes of these independent education activities, best efforts were undertaken to ensure compliance with the PHAP code, however, you should review your local prescribing information and consult directly with Novartis Healthcare Philippines, Inc. to address any questions
Prescribing information may vary depending on local health authority approval in each country. Before prescribing any product, always refer to the Summary of Product Characteristics (SmPC) or product information approved in your local country.